The status of members of Phylum Annelida and related phyla has been under scrutiny. Membership of Annelida has varied since its inception as a group emerging from the Vermes in the early 19th century. In recent times the general view is that it contains polychaetes and the clitellate oligochaetes and leeches, usually as three classes, or latterly with the clitellates as one class or sub-phylum. But we may wonder if the many and diverse polychaete families are a monophyletic group or a miscellaneous hodge-podge? Are the oligochaetes just a small part of the polychaetes? Should the category 'polychaete' be used at all? Can it be defined? What about other worms with setae? Should the vestimentiferans and pogonophorans be sister phyla or included in the polychaetes? What about the echiurans? And what are the major groupings of the protostomes?
Winnepenninckx, B.; Backeljau, T.; De Wachter, R. (1995)
Phylogeny of protostome worms
derived from 18S rRNA sequences.
Molecular Biology and Evolution 12(4): 641-
649.
Abstract: The phylogenetic relationships of protostome worms were studied by comparing new complete 18S rRNA sequences of [mostly single taxa of] Vestimentifera, Pogonophora, Sipuncula, Echiura, Nemertea, and Annelida with existing 18S rRNA sequences of Mollusca, Arthropoda, Chordata, and Platyhelminthes. Phylogenetic trees were inferred via neighbor-joining and maximum parsimony analyses. These suggest that (1) Sipuncula and Echiura are not sister groups; (2) Nemertea are protostomes; (3) Vestimentifera and Pogonophora are protostomes that have a common ancestor with Echiura and (4) Vestimentifera and Pogonophora are a monophyletic clade.
Nielsen, C. (1995 (1997))
Animal evolution. Interrelationships of the living
phyla
Oxford, Oxford University Press. 467 pp.
[Chapter 17, Phylum Annelida (p. 126- 148) includes commentary on features of clitellates, pogonophorans, echiurans, etc, that may relate to their phylogenetic position relative to the polychaetes. "... the word 'polychaetes' can be used as a colloquial term for the less specialized annelds, but it has no place in the systematic vocabulary." Jenner & Schram (1999) have criticisms of Nielsen's approach in this book and in a subsequent fully cladistic analysis.]
Rouse GW; Fauchald K. (1995)
The Articulation of Annelids.
Zoologica
Scripta, , 24(4) (OCT): 269-301
Abstract: The aim of this paper is to assess the monophyly of the Annelida. Also, recent
cladistic analyses of metazoan taxa, using a variety of data, have shown incongruities with
regards to annelids and associated taxa that should be resolved. The Platyhelminthes is selected
as the taxon to root our minimal length trees and polarise our characters in a parsimony analysis;
ingroup taxa being Mollusca, Nemertea, Sipuncula, Echiura, Pogonophora, Vestimentifera,
Euarthropoda, Onychophora, and the groups most commonly regarded as true 'annelids', the
Clitellata and Polychaeta. We use 13 characters and a total of 33 states. This results in 18
minimal length trees of 23 steps. The consensus tree has the topology (Platyhelminthes (Nemertea
(Sipuncula Mollusca (Echiura (Polychaeta (Vestimentifera Pogonophora) Clitellata (Euarthropoda
Onychophora)))))).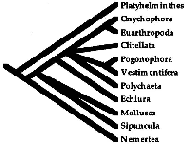
The name Articulata is applied to the Clitellata, Euarthropoda, Onychophora, Pogonophora,
Polychaeta, and Vestimentifera. The Vestimentifera is the sister group to, or more likely a clade
within, the frenulate pogonophores, and the name Pogonophora is retained for this group. In half
of the 18 minimal length trees, the traditionally formulated Annelida, i.e. Polychaeta and
Clitellata, is paraphyletic if the Pogonophora are excluded. In the remaining minimal length
trees, a monophyletic Annelida cannot be formulated. The name Annelida should not be used unless
relationships within the Articulata are resolved to show it is a monophyletic taxon.
The taxon name Articulata, originally formulated to include the Annelida and Arthropoda by Cuvier,
is defined as the clade stemming from the first ancestor to show repetition of homologous body
structures derived by teloblastic growth with a pygidial growth zone (segmentation) and
longitudinal muscles broken into bands. The Articulata is considered, on current evidence, to
consist of four monophyletic groups; the Arthropoda, Clitellata, Polychaeta, and Pogonophora,
though the latter group may be a clade of polychaetes. If this is shown, the Pogonophora should
revert to the original family name Lamellisabellidae Uschakov, 1933. An indented classification
reflective of the cladistic pattern is provided. Other recent hypotheses about metazoan
systematics are analysed.
(Original drawing ©1995 by The Norwegian Academy of Sciences and Letters)
[Commentaries on this morphological paper and on the value of molecular papers with limited
sequence data ("To sequence, say, a couple of flatworms, a sipunculan, a
vestimentiferan, a polychaete, an oligochaete, and a fruit fly, and produce
a cladogram in the name of gaining substantive understanding of phylum
relationships is laughable.") appear in the May 1996 archive of Annelida list.
The
authors included lengthy reviews of the history of some groups, including Pogonophora and
Vestimentifera. Rouse and Fauchald in a later paper retracted Lamellisabellidae Uschakov, 1933 and
substituted Siboglinidae Caullery, 1914 as the proposed name for pogonophorans inside Annelida.
Jenner and Schram (1999) have pointed out how the result of R & F's
analysis hinges on one coding decision]
Kim, C. B.; Moon, S. Y.; Gelder, S., R.; Kim, W. (1996)
Phylogenetic relationships of
annelids, molluscs, and arthropods evidenced from molecules and morphology.
Journal of
Molecular evolution 43207-215.
[18S ribosomal RNA data for single taxa representatives of the groups gives monophyly for both annelids and molluscs, and a sister relationship between the two.]
Agiunaldo, A. M. A.; Turbeville, J. M.; Linford, L. S.; Rivera, M. C.; Garey, J. R.; Raff, R.
A.; Lake, J. A. (1997)
Evidence for a clade of nematodes, arthropods and other moulting
animals.
Nature 387( 6632 (05-29-1997) letters to nature): 489-492.
Abstract: The arthropods constitute the most diverse animal group, but, despite their rich fossil record and a century of study, their phylogenetic relationships remain unclear. Taxa previously proposed to be sister groups to the arthropods include Annelida, Onychophora, Tardigrada and others, but hypotheses of phylogenetic relationships have been conflicting. For example, onychophorans, like arthropods, moult periodically, have an arthropod arrangement of haemocoel, and have been related to arthropods in morphological and mitochondrial DNA sequence analyses. Like annelids, they possess segmental nephridia and muscles that are a combination of smooth and obliquely striated fibres. Our phylogenetic analysis of 18S ribosomal DNA sequences indicates a close relationship between arthropods, nematodes and all other moulting phyla. The results suggest that ecdysis (moulting) arose once and support the idea of a new clade, Ecdysozoa, containing moulting animals: arthropods, tardigrades, onychophorans, nematodes, nematomorphs, kinorhynchs and priapulids. No support is found for a clade of segmented animals, the Articulata, uniting annelids with arthropods. The hypothesis that nematodes are related to arthropods has important implications for developmental genetic studies using as model systems the nematode Caenorhabditis elegans and the arthropod Drosophila melanogaster, which are generally held to be phylogenetically distant from each other.
[The sister protostome clade, the Lophotrochozoa included annelids, molluscs, rotifers, phoronids, brachiopods, bryozoans, platyhelminthes and related phyla.]
Purschke G (1997):
Ultrastructure of nuchal organs in polychaetes (Annelida) - New
results and review.
Acta Zoologica 78(2), 123-143.
Abstract: Nuchal organs are epidermal sensory structures present in most polychaetes. They are situated at the posterior edge of the prostomium and may extend posteriorly onto the peristomium. Although there is considerable external variation, they all consist of ciliated supporting cells, bipolar primary sensory cells and retractor muscles. They are innervated directly from the brain by paired nerves. The sensory cells are usually monociliated; their sensory processes lie in subcuticular spaces, the olfactory chambers. Structural variability is to be observed in the location of the sensory cells, the course of the nuchal nerve, position of nuchal ganglia as well as in cytological features of sensory and supporting cells. These differences provide useful characters for phylogenetic considerations to establish supraspecific taxa within the phylogenetic system of the Annelida. Special emphasis is laid on the problem of whether the nuchal organs represent an autapomorphy of the Polychaeta or the Annelida and thus whether the lack of nuchal organs in Clitellata is primary or secondary. As is discussed, the probability of a loss of the nuchal organs in Clitellata is higher, which favours the second hypothesis: that nuchal organs are part of the ground pattern of the Annelida and very likely are an autapomorphy of this group.
Eibye-Jacobsen,Danny; Nielsen,Claus (1997 (1996)
Point of view. The
rearticulation of annelids.
Zoologica Scripta. 25(3), 275-282.
Some salient extracts: "The main objective of this paper is to discuss the profound influence the choice of input taxa has on the results of a cladistic analysis, provide an alternative interpretation of Rouse and Fauchald's [1995] data , and ultimately take issue with their conclusion regarding the monophyly of Annelida." [After demonstrating ambiguity caused by including paraphyletic groups in cladistic analysis the authors reanalyzed Rouse and Fauchald's data, removing the Pogonophora and Vestimentifera on the assumption that they form a monophyletic subclade within Polychaeta, and in another analysis removing Echiura (also within Polychaeta). None of the trees generated supported a clade in which Polychaeta and Clitellata were separated.] "... in our opinion the crucial issue is chaetation [not segmentation]. ... We furthermore hope that coming investigations will consider the possibility the Clitellata is in fact an ingroup of the Polychaeta, which would make Annelida and Polychaeta synonymous."
Rouse,G. (1997)
Point of view. Rearticulating with extra assumptions; a response to
Eibye-Jacobsen and Nielsen.
Zoologica Scripta 26(1), 61-66.
Some salient extracts: [argues that points raised are] "either incorrect, irrelevant or make additional assumptions ..." [Concludes that] "They require several additional assumptions to 'keep' the Annelida, and one wonders why they bothered. What is required is further evidence and study now that the possibility of the paraphyly of the Annelida has been raised."
[A further exchange then took place on ANNELIDA list and may be read in the December 1997 archive.]
Westheide, W. (1997)
The direction of evolution within the polychaeta.
Journal of Natural History. 31(1): 1-15.
Abstract: The phylogenetic systematics of the polychaetes, i.e. the nonclitellate annelids, depend on which characters are regarded as belonging to the bauplan of the annelid stem species. The main competition is between two diametrically opposed hypotheses: the stem species was either (1) an errant, epibenthic organism with well- developed prostomium and prostomial appendages (antennae and palps), many homonomous segments, biramous and well differentiated parapodia, and numerous well-structured chaetae, or (2) a burrowing organism with small prostomium lacking appendages, which had many homonomous segments without parapodia, and only a few simple chaetae. (A third hypothesis, which is based primarily on certain morphological peculiarities and the presence of exclusively monociliary cells in Owenia, and which postulates a sessile stem species, is mentioned only peripherally: for the present.) From a decision in favour of Hypothesis 2 it would follow that the Clitellata should be considered the most primitive annelids, so that the possession of parapodia and many extremely differentiated chaetae, for instance, would be interpreted as a highly derived character state. The consequence for the phylogenetic systematics of the Polychaeta is that oligochaete-like taxa would have to be considered more primitive than, for example, nereidid- like taxa. On the basis of Hypothesis 1, the evolution of these structures would have proceeded in the opposite direction, and polychaete systematics would have the reverse arrangement. The most important evidence for Hypothesis 2 comes from functional morphological considerations; namely the inference that metamerism has arisen from a burrowing mode of life. It is shown here that (1) this hypothesis rests partly on ignorance of the close relationship between reproductive biology and morphology in the clitellates, (2) the notion that metamerism, and hence the stem species of the Articulata, originated from a burrowing life in the marine environment is unconvincing, and (3) the origin of metamerism can be explained quite differently with reference to modern ultrastructural findings. According to these findings, septa, which are the fundamental structural elements for annelid segmentation, evolved as a morphological prerequisite for the development of transversely running blood vessels; other purposes of septa (e.g. subdivision of the hydrostatic skeleton), therefore, have to be regarded as secondary. A highly complex blood vascular system may have been the consequence of the development of lateral parapodia-like appendages. Thus, parapodia are assumed to be part of the ground pattern of the Articulata and hence were present in the stem species of the Annelida. This is consistent with the traditional interpretation of annelid systematics, which places the errant polychaete taxa at the base of the system (Hypothesis 1).
McHugh, Dahmnait (1997) Molecular evidence that echiurans and pogonophorans are derived annelids. Proceedings of the National Academy of Sciences USA 94: 8006-8009 - (Online or Reprint online in PDF format))
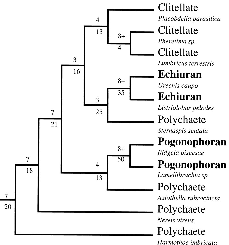 Abstract: The Annelida, which includes the polychaetes and the clitellates, has
long held the taxonomic rank of phylum. The unsegmented, mud-dwelling echiuran spoon worms and the
gutless, deep-sea pogonophoran tube worms (including vestimentiferans) share several embryological
and morphological features with annelids, but each group has also been considered as a separate
metazoan phylum based on the unique characters they display. Phylogenetic analyses of DNA
sequences from the nuclear gene elongation factor-1alpha place echiurans and pogonophorans within
the Annelida. This result, indicating the derived loss of segmentation in echiurans, has profound
implications for our understanding of the evolution of metazoan body plans, and challenges the
traditional view of the phylum-level diversity and evolutionary relationships of protostome
worms.
Abstract: The Annelida, which includes the polychaetes and the clitellates, has
long held the taxonomic rank of phylum. The unsegmented, mud-dwelling echiuran spoon worms and the
gutless, deep-sea pogonophoran tube worms (including vestimentiferans) share several embryological
and morphological features with annelids, but each group has also been considered as a separate
metazoan phylum based on the unique characters they display. Phylogenetic analyses of DNA
sequences from the nuclear gene elongation factor-1alpha place echiurans and pogonophorans within
the Annelida. This result, indicating the derived loss of segmentation in echiurans, has profound
implications for our understanding of the evolution of metazoan body plans, and challenges the
traditional view of the phylum-level diversity and evolutionary relationships of protostome
worms.
Extracts: "If echiurans are in fact modified annelids and do not represent the unique body plan that their phylum status implies, then segmentation is an evolutionarily labile body plan character that has been lost rather than never gained by echiurans." "[Tree analysis gives] echiurans, the pogonophorans, and the clitellates ... within a paraphyletic grade of polychaetes." "Retention of the taxon Polychaeta is not logically consistent with the phylogeny presented here, and it is proposed that the term 'polychaete' be used only as an informal reference for a grade of marine annelids." "As clades within the Annelida, the echuirans revert to therir original name, the Echiuridae, and the pogonophorans assume the name erected in the first species description for the group, the Siboglinidae."
[See Siddall et al. (1998) for a highly critical view of this paper]
[ Complete consensus tree image ] (Drawing ©1997 by The National Academy of Sciences of the USA). Fauchald,K; Rouse,GW (1997)
Polychaete systematics: past and present.
Zoologica Scripta 26(2): 71-138.
Abstract: In this paper, we first demonstrate the historical background
for the current unsatisfactory state of systematics of the polychaetes. We then briefly discuss
our knowledge of internal and external structures. A review of the polychaete families makes up
the third section; 81 families are treated in detail. Five families have been recently synonymized
with others, and six families are too poorly known to be sufficiently characterized. Fossil
polychaetes are briefly mentioned, with specific attention to problems associated with
incorporating them in recent systematics.
The traditional separation in 'errant' and 'sedentary' polychaetes has increasingly become
recognized as being unsatisfactory; however, the current trend towards grouping the polychaetes in
many orders without specifying the relationships among the orders, is no more satisfactory. The
lack of consistent morphological information is a major source of uncertainty. Intensive
morphological studies should remove terminological ambiguities and alleviate some of the problems.
Rouse,GW; Fauchald,K.(1997)
Cladistics and polychaetes.
Zoologica Scripta 26(2): 139-204.
Abstract: A series of cladistic analyses assess the status and membership of the
taxon Polychaeta. The available literature, and a review by Fauchald & Rouse (1997), on the 80
accepted families of the Polychaeta is used to develop characters and data matrices. As well as
the polychaete families, non- polychaete taxa such as the Echiura, Euarthropoda, Onychophora,
Pogonophora (as Frenulata and Vestimentifera), Clitellata, Aeolosomatidae and Potamodrilidae are
included in the analyses. All trees are rooted using the Sipuncula as outgroup. Characters are
based on features (where present) such as the prostomium, peristomium, antennae, palps, nuchal
organs, parapodia, stomodaeum, segmental organ structure and distribution, circulation and
chaetae. A number of analyses are performed involving different ways of coding and weighting the
characters, as well as the number of taxa included. Transformation series are provided for several
of these analyses. One of the analyses is chosen to provide a new classification.
The Annelida is found to be monophyletic, though weakly supported, and comprises the Clitellata
and Polychaeta. The Polychaeta is monophyletic only if taxa such as the Pogonophora,
Aeolosomatidae and Potamodrilidae are included and is also weakly supported. The Pogonophora is
reduced to the rank of family within the Polychaeta and reverts to the name Siboglinidae Caullery,
1914. The new classification does not use Linnaean categories and the Polychaeta comprises two
clades, the Scolecida and Palpata. The Palpata has the clades Aciculata and Canalipalpata. The
Aciculata contains the Phyllodocida and Eunicida. The Canalipalpata has three clades; the
Sabellida (including the Siboglinidae) Spionida and Terebellida. The position of a number of
families requires further investigation.
[ Data matrices at Sydney Uni. ] [Table of families ] - (The latter is a link to the Polychaeta higher
classification page here.)
In the chapter, The Annelida. Rouse, G. 1999. p. 174-203,
In: D. T. Anderson (ed.), Invertebrate Zoology, Oxford University Press, Australia, Melbourne (467
pp).
Rouse presents the same higher classification, incorporates the pogonophorans as Family
Siboglinidae within the polychaetes, and has Echiura retained outside annelids meantime.
Kojima,Shigeaki (1998)
Paraphyletic status of Polychaeta suggested by
phylogenetic analysis based on the amino acid sequences of elongation
factor-1 alpha.
Molecular Phylogenetics and Evolution 9(2): 255-261.
Abstract: In order to judge whether or not Polychaeta is a paraphyletic group, I determined almost the entire amino acid sequence of elongation factor-l alpha from thirteen polychaetes, two oligochaetes, two hirudineans, two vestimentiferans, and two molluscs. Phylogenetic analysis by the neighbor-joining (NJ) method and the maximum likelihood (ML) method indicated the monophyly of Clitellata (the oligochaetes and hirudineans). In both the NJ and ML trees, vestimentiferans and clitellates were derived from polychaetes independently. The present results strongly suggest that Polychaeta is a paraphyletic group.
Rouse Greg, W. K. Fauchald (1998)
Recent views on the status, delineation and classification of the Annelida.
American
Zoologist 38:953-964.
Abstract: In recent years the monophyly of the Annelida and Polychaeta has been questioned, with various authors proposing that groups such as the Arthropoda, Echiura and Pogonophora render the Annelida and/or Polychaeta paraphyletic. The Clitellata have also been proposed to be a member of the Polychaeta, potentially making this latter taxon synonymous with the Annelida. The relationships within the traditionally formulated Polychaeta have never been investigated using cladistic methodology. Recent classifications of polychaetes show a large number of "orders" with no real attempts to relate the groups in a phylogenetic sense. In this paper a number of recent studies on annelid systematics and classification are reviewed. Special emphasis is placed on the cladistic parsimony analyses of Rouse and Fauchald (1995, 1997) where a comprehensive assessment of the relationships among the various polychaete and annelid groups was attempted. A contrasting result by Westheide (1997) using a different methodology, is also outlined and discussed.
McHugh, D.; G. W. Rouse (1998)
Life history evolution of marine invertebrates: New views
from phylogenetic systematics.
Trends in Ecology & Evolution 13:182-186.
Abstract: Established theories on the evolution of the diverse life histories of marine metazoans, specifically invertebrates, were developed in the absence of rigorous phylogenetic methods. With improved estimates of evolutionary relationships for various marine invertebrate groups, based on phylogenetic systematics, we can now critically evaluate the assumptions upon which these theories are based. Several studies emphasizing a phylogenetic systematics approach have recently examined the evolutionary transitions among reproductive traits and challenge us to reconsider the generality of the assumptions made about life history evolution. The results point towards exciting possibilities for a better understanding of the great diversity of reproductive and developmental modes we observe in marine invertebrates today.
Halanych, K. M., R. A. Lutz, & R. C. Vrijenhoek. (1998)
Evolutionary origins and age of
vestimentiferan tube-worms.
Cahiers de Biologie Marine, 39:355-358.
Abstract: Vestimentiferan tube-worms are one of the dominant groups of organisms present at deep-sea hydrothermal vent habitats in the eastern Pacific Ocean. Understanding how they have evolved to thrive in such harsh environments is a subject of great interest to marine biologists. In order to assess the degree and polarity of this evolutionary change, we have used a molecular phylogenetic approach to examine the age and history of the vestimentiferans. Considerable debate persists concerning the taxonomic status and evolutionary origins of vestimentiferans. Jones (1985) argued that the vestimentiferan body plan was sufficiently distinct to warrant placement in a unique phylum, the Vestimentifera. On the other hand, Southward (1988), among others, challenged the erection of a separate phylum for these worms. During the course of recent taxonomic debates, various authors have also referred to these worms as the Order Vestimentifera (Class Afrenulata: Phylum Pogonophora) and the Subclass Obturata (Class Pogonophora: Phylum Annelida). Although the rationale behind such changes in taxonomic rank may be legitimate, higher taxonomic categories are manmade constructs that are not meaningful for describing the diversity or the age of a monophyletic clade of organisms. To avoid assigning rank to taxonomic names throughout this manuscript, we use the terms "vestimentiferan" and "perviate pogonophoran" (i.e., the traditional non-vestimentiferan pogonophorans). To date, our work has focused on partial sequences from the mitochondrial cytochrome c oxidase subunit 1 (CO1) gene (Black et al., 1997), sequences from the nuclear small ribosomal subunit (18S rRNA), and a 3' region of the large ribosomal subunit (28S rRNA). The present molecular data provide support for the inclusion of perviate pogonophorans and vestimentiferans as a clade within a paraphyletic grade of traditional annelid taxa. Furthermore, extant vestimentiferans appear to be a relatively young group that radiated during the Cenozoic.
Abouheif, E.; Zardoya, R.; Meyer, A. (1998)
Limitations of metazoan 18S rRNA sequence
data: implications for reconstructing a phylogeny of the animal kingdom and inferring the reality
of the Cambrian explosion.
Journal of Molecular Evolution 47: 394-405.
Abstract: We document the phylogenetic behavior of the 18S rRNA molecule in 67 taxa from 28 metazoan phyla and assess the effects of among-site rate variation on reconstructing phylogenies of the animal kingdom. This empirical assessment was undertaken to clarify further the limits of resolution of the 18S rRNA gene as a phylogenetic marker and to address the question of whether 18S rRNA phylogenies can be used as a source of evidence to infer the reality of a Cambrian explosion. A notable degree of among-site rate variation exists between different regions of the 18S rRNA molecule, as well as within all classes of secondary structure. There is a significant negative correlation between inferred number of nucleotide substitutions and phylogenetic information, as well as with the degree of substitutional saturation within the molecule. Base compositional differences both within and between taxa exist and, in certain lineages, may be associated with long branches and phylogenetic position. Importantly, excluding sites with different degrees of nucleotide substitution significantly influences the topology and degree of resolution of maximum-parsimony phylogenies as well as neighbor-joining phylogenies (corrected and uncorrected for among-site rate variation) reconstructed at the metazoan scale. Together, these data indicate that the 18S rRNA molecule is an unsuitable candidate for reconstructing the evolutionary history of all metazoan phyla, and that the polytomies, i.e., unresolved nodes within 18S rRNA phylogenies, cannot be used as a single or reliable source of evidence to support the hypothesis of a Cambrian explosion.
Siddall,Mark E; Fitzhugh,Kirk; Coates,Kathryn A (1998)
Problems Determining the Phylogenetic Position of Echiurans and Pogonophorans
with Limited Data.
Cladistics 14(4), 401-410.
Abstract: The phylogenetic position of Echiura and Pogonophora was recently reconsidered using 346 bp of elongation factor 1-alpha (EF-1). On the basis of these results alone it was suggested that the relationships of Annelida be reconsidered and that the systematics of the group changed accordingly. An examination of these data, however, reveals difficulties in the original analysis that relate to weighting and the relative inclusion or exclusion of available characters and of taxa. Wholesale revision of the systematics of Annelida or of groups therein awaits the availability of more comprehensive work.
Comments. This paper is a critique of McHugh (1997) (see above) and a re-analysis with extra taxa. The authors, albeit in a rather strident tone, make a convincing case that McHugh's conclusions are premature and unsupported by the data (although they allow that the proposition may well be true). The Siddall paper does not follow the good example of McHugh in that it surprisingly fails to identify beyond genus-level the species in the analysis, and does not indicate the data sources.
McHugh, D. (1999)
Phylogeny of the
Annelida: Siddall et al. (1998) Rebutted.
Cladistics 15(1), 85-89.
McHugh's response is outlined in her opening paragraph: "Siddall et al. (1998) recently published a critique of my phylogenetic analysis of annelid, echiurid, and siboglinid (= Pogonophora and Vestimentifera (see McHugh, 1997; Rouse and Fauchald, 1997)) relationships based on the nuclear coding gene, elongation factor-1a (EF-1a) (McHugh, 1997). Unfortunately, Siddall et al. (1998) made several misleading and obfuscatory statements about the EF-1a analysis, and omitted information that supports the EF-1a results that I presented. In addition, they stated that they could not reproduce the results of McHugh (1997), which may give readers the mistaken impression of discordance between the methods I applied and the results I presented. Here, to clarify matters and to avoid the propagation of incorrect information, I directly address specific quotes (in italics) from Siddall et al. (1998) in order of their appearance in that manuscript."
McHugh intimated further unpublished analyses with more taxa and sequence data continue to support the original paper.
Rouse, Greg W. (1999)
Trochophore concepts: ciliary bands and the evolution of larvae in spiralian Metazoa
Biological Journal of the Linnean Society 66(4):411-464
Abstract: "Trochophore" is a term used in a strict sense for larvae having an opposed- band method of feeding, involving a prototroch and metatroch. Other ciliary bands such as a telotroch and neurotroch may be present. The trochophore has been proposed to represent the ancestral larval form for a group of metazoan phyla (including all members of the Spiralia). The name trochophore is also often applied to larvae that do not conform to the above definition. A cladistic analysis of spiralian taxa (with special reference to polychaete annelids), based on a suite of adult and larval characters, is used to assess several hypotheses: (1) that the trochophore (in a strict sense) is a plesiomorphic form for the Spiralia; (2) that the strictly defined trochophore is plesiomorphic for members of the Spiralia such as the Polychaeta. The homology of each of the various separate ciliary bands of spiralian larvae, and features such as the apical tuft and protonephridia is also assessed. The results favour the conclusion that the trochophore, if defined as a feeding larval form using opposed bands, should not be regarded as an ancestral (= plesiomorphic) type for the Spiralia, or any other large taxon such as the Polychaeta or Mollusca. The evidence suggests that the various ciliary bands have differing evolutionary histories, and only the Echiura (possibly an annelid group) has members with the classical trochophore. The trochophore is re-defined as a larval form with a prototroch. This broad definition covers a wide variety of larvae, and matches the current usage more accurately than the restricted term. Features such as the neurotroch, telotroch and opposed-band feeding show convergence and reversals. The nature of the metatroch requires further investigation. The presence of a prototroch (and hence trochophore larvae) is used to identify an apomorphy-based taxon, Trochozoa, that includes the first ancestor to have evolved a prototroch and all its descendants. This minimally includes the Annelida (sensu lato), Echiura, Entoprocta, Mollusca and Sipuncula and is a less inclusive taxon than the Spiralia.
[Further data, including the matrix, is online at http://wallace.bio.usyd .edu.au/papers/gregr/Trochophore/Trocho1.htm]
Comments.This paper is an extension of Rouse and Fauchald 1997. It adds on 16 larval morphology characters to the 124 characters of the first analysis. The characters are mainly cilia bands. It corrects some errors in the original 1997 matrix (see p. 418), places some families in different clades than previously (notably Chaetopteridae moved to Sabellida, not Spionida, and Oweniidae moved from Sabellida to a clade with Polygordiidae, etc), and has a few scoring errors of its own (note added in proof).
Jenner, R. A.; Schram, F. R. (1999)
The grand game of metazoan
phylogeny: rules and strategies.
Biological Reviews 74(2): 121-142.
[This paper is highly critical of all phylogeny papers to date derived from morphological cladistics. For example it is pointed out that Rouse and Fauchald's (1995) support for the Articulata fails if the data is reanalysed with an arguable different coding for just one character - cuticle type - for one taxon, the molluscs.]
Boore, J. L.;Brown, W. M. (2000)
Mitochondrial genomes of Galathealinum,
Helobdella, and Platynereis: Sequence and gene arrangement comparisons indicate that
Pogonophora is not a phylum and Annelida and Arthropoda are not sister taxa.
Molecular
Biology and Evolution 17(1):87-106.
Abstract: We report a contiguous region of more than half (>7,500 nt) of the mitochondrial genomes for Platynereis dumerii [sic for dumerilii] (Annelida: Polychaeta), Helobdella robusta (Annelida: Hirudinida), and Galathealinum brachiosum (Pogonophora: Perviata). The relative arrangements of all 22 genes identified for Helobdella and Galathealinum are identical to one another and to their arrangements in the mtDNA of the previously studied oligochaete annelid Lumbricus. In contrast, Platynereis differs from these taxa in the positions of several tRNA genes and in having two additional tRNA genes (trnC and trnM) and a large noncoding sequence in this region. Comparisons of relative gene arrangements and of the nucleotide and inferred amino acid sequences among these and other published taxa provide strong support for an annelid-mollusk clade that excludes arthropods, and for the inclusion of pogonophorans within Annelida, rather than giving them separate phylum status. Gene arrangement comparisons include the first use of a recently described method on previously unpublished data. Although a variety of alternative initiation codons are typically used by mitochondrial protein-encoding genes, ATG appears to be the initiator for all but one reported here. The large noncoding region (1,091 nt) identified in Platynereis has no significant sequence similarity to the noncoding region of Lumbricus, although each contains runs of TA dinucleotides and of homopolymers, which could potentially serve as signaling elements. There is strong bias for synonymous codon usage in Helobdella and especially in Galathealinum. In this latter taxon, 5 codons are completely unused, 13 are used three or fewer times, and G appears at third codon positions in only 26 of the 2,236 codons. Nucleotide composition bias appears to influence amino acid composition of the proteins.
Gerhart, J. (2000)
Inversion of the chordate body axis: Are there
alternatives?
Proceedings of the National Academy of Sciences 97(9):4445-4448.
Abstract: One major morphological difference between chordates and annelids or arthropods is the opposite orientation of the nerve cord and heart. A long-standing proposal is that the chordate axis evolved by inverting the body of an ancestor with the annelid/arthropod orientation. However, the data can also be explained by a common ancestor with diffuse dorsoventral organization, followed by oppositely directed condensation of the nerve cord and relocation of the heart in the two lines.
Eeckhaut, I.; McHugh, D.; Mardulyn, P.; Tiedemann, R.; Monteyne, D. M.; Jangoux, M.; &
Milinkovitch, M. C. (2000)
Myzostomida: a link between trochozoans and flatworms?
Proceedings of the Royal Society London, B 267(1451):1383-1392.
Abstract: Myzostomids are obligate symbiotic invertebrates associated with echinoderms with a fossil record that extends to the Ordovician period. Due to their long history as host- specific symbionts, myzostomids have acquired a unique anatomy that obscures their phylogenetic affinities to other metazoans: they are incompletely segmented, parenchymous, acoelomate organisms with chaetae and a trochophore larva. Today, they are most often classified within annelids either as an aberrant family of polychaetes or as a separate class. We inferred the phylogenetic position of the Myzostomida by analysing the DNA sequences of two slowly evolving nuclear genes: the small subunit ribosomal RNA and elongation factor-1alpha. All our analyses congruently indicated that myzostomids are not annelids but suggested instead that they are more closely related to flatworms than to any trochozoan taxon. These results, together with recent analyses of the myzostomidan ultrastructure, have significant implications for understanding the evolution of metazoan body plans, as major characters (segmentation, coeloms, chaetae and trochophore larvae) might have been independently lost or gained in different animal phyla.
Purschke, G.; Hessling, R. ; Westheide, W. (2000)
The phylogenetic position of the
Clitellata and Echiura - on the problematic assessment of absent characters.
Journal of
Zoological Systematics & Evolutionary Research 38(3):165-173.
Abstract: Absent characters (negative characters) are difficult to assess and their correct interpretation as symplesiomorphies, synapomorphies or convergencies (homoplasies) is one of the greatest challenges in phylogenetic systematics. Different phylogenetic assessments often result in contradictory phylogenetic hypotheses, in which the direction of evolutionary changes is diametrically opposed. Especially in deciding between primary (plesiomorphic) and secondary (apomorphic) absence, false conclusions may be reached if only the outgroup comparison and the principle of parsimony are employed without attempting any biological evaluation or interpretation of characters. For example, in the higher-level systematization of the Annelida and related taxa different assessments of absent characters have led to conflicting hypotheses about the phylogenetic relationships and the ground pattern of the annelid stem species. Varying phylogenetic interpretations regarding the absence of the chemosensory nuchal organs in the clitellates and their presence in polychaetes initiated a controversy that produced two alternative phylogenetic hypotheses: (1) the Clitellata are highly derived Annelida related to a subtaxon within the, in this case, paraphyletic "Polychaeta" or (2) the Clitellata are comparatively primitive Annelida representing the sister group of a monophyletic taxon Polychaeta. In the former, the absence of nuchal organs in the Clitellata is regarded as a secondary character, in the latter as primary. Since most Clitellata are either limnetic or terrestrial, we must ask which characters are plesiomorphies, taken from their marine stem species without changes. Besides a thorough investigation and evaluation of clitellate characters, a promising approach to these questions is to look for such characters in limnetic and terrestrial annelids clearly not belonging to the Clitellata. A similar problem applies to the evaluation of the position of the Echiura, which lack both segmentation and nuchal organs. Evidence is presented that in both taxa these absent characters represent derived, apomorphic character states. The consequences for their phylogenetic position and the questionable monophyly of the Polychaeta are discussed. The conclusion drawn from morphological character assessments is in accordance with recently published hypotheses based on molecular data.
Valentine, J. W.; Collins, A. G. (2000)
The significance of moulting in Ecdysozoan
evolution.
Evolution & Development 2(3):152-156.
Abstract: Three major bilaterian clades first appear in the Early Cambrian fossil record: Deuterostomia, Lophotrochozoa, and Ecdysozoa. The taxa placed in Ecdysozoa are characterized by a moulting habit, unknown in the other major clades. The origin and consequences of moulting are of fundamental importance to the history of the ecdysozoan clade, chiefly because moulting precludes motile ectodermal cilia. Moulting may have originated as an adaptation to permit the enlargement, during growth, of secreted cuticular spines, flanges, and other structures used as ancillary locomotory devices. A combination of phylogenetic and fossil evidence suggests that the early members of these clades were small vermiform paracoelomates that likely lacked indirect-developing planktotrophic larvae. Thus, the evolution of planktotrophic larvae may have been independently achieved at least three times within Bilateria. The nonmoulting clades evolved larvae that swim and feed via ciliated tufts and bands, presumably intercalating these forms within their early developmental systems. Within Ecdysozoa, feeding larvae lacked ciliary feeding tracts and evolved by modification of early instars, employing limbs or setae to generate feeding currents. The setting aside during larval life of cells that give rise to adult features is probably an adaptation associated with metamorphosis.
So do Echiura, Vestimentifera, and Pogonophora still exist as independent phyla?
It is still undecided whether the Echiura are in Annelida, but it is probable that the vent worms and pogonophorans should be combined, and considered annelids, perhaps at family level, perhaps higher. However, the stance of 'Zoological Record' on proposals to change phylum status, etc should be noted. It records the proposals under the original phyla names, but is otherwise unmoved until it is apparent the change has been adopted widely.
If Echiura is indeed moved into Annelida it should also be noted that there is a case that the name Echiuridae as proposed by McHugh may be invalid as a junior synonym to Thalassematidae, although in the new, year 2000, code a new article, 35.5. Precedence [post 1999] for [family-group] names in [prevailing] use at higher rank, will mean Echiuridae will become safe to use as long as Echiuridae and Thalassematidae remain synonyms at family-group level. [ see "Re: The fate of the early adopters" and related messages in Annelida archive. ]
And where do the annelids belong within metazoa?
The groupings of protostome metazoan phyla remain unresolved. At present no consensus looks imminent.
The issues of the derivation of segmentation and of limb development are topical in evolutionary biology. Also certain Hox genes are shared by subsets of protostome phyla.
Pennisi,Elizabeth; Roush,Wade (1997):
Developing a new view of
evolution. Science 277(4 July), 34-37.
Panganiban,G; Irvine,SM; Lowe,C; Roehl,H;
Corley,LS; Sherbon,B; Grenier,JK; Fallon,JF; Kimble,J; Walker,M; Wray,GA; Swalla,BJ;
Martindale,MQ; Carroll,SB (1997):
The origin and evolution of animal appendages.
Proceedings of the National Academy of Sciences of the United States of America 94(10),
5162-5166.) [Online copy][PDF reprint].
de Rosa, R.; Grenier, J. k.; Andreeva, T.; Cook, C. e.; Adoutte, A.; Akam, M.; Carroll, S. B.;
Balavoine, G. (1999)
Hox genes in brachiopods and priapulids and protostome
evolution.
Nature 399(24 June 1999): 772-776.